Published in Metabolic Engineering, Volume 91, 2025, Pages 103-118, ISSN 1096-7176.
https://doi.org/10.1016/j.ymben.2025.03.011
Highlights
- Genome-scale metabolic models for HEK293 strains are reconstructed.
- A time-resolved biomass composition dataset for HEK293 strains is provided.
- Theoretical analysis suggests HEK293 could produce 100 times more AAV.
- HIF-1 activity limits AAV production in the low-producing strain.
- Inhibiting HIF-1 halts growth and boosts AAV capsid production by 2.5-fold.
Abstract
HEK293 cells are a versatile cell line extensively used in the production of recombinant proteins and viral vectors, notably Adeno-associated virus (AAV) (Bulcha et al., 2021). Despite their high transfection efficiency and adaptability to various culture conditions, challenges remain in achieving sufficient yields of active viral particles.
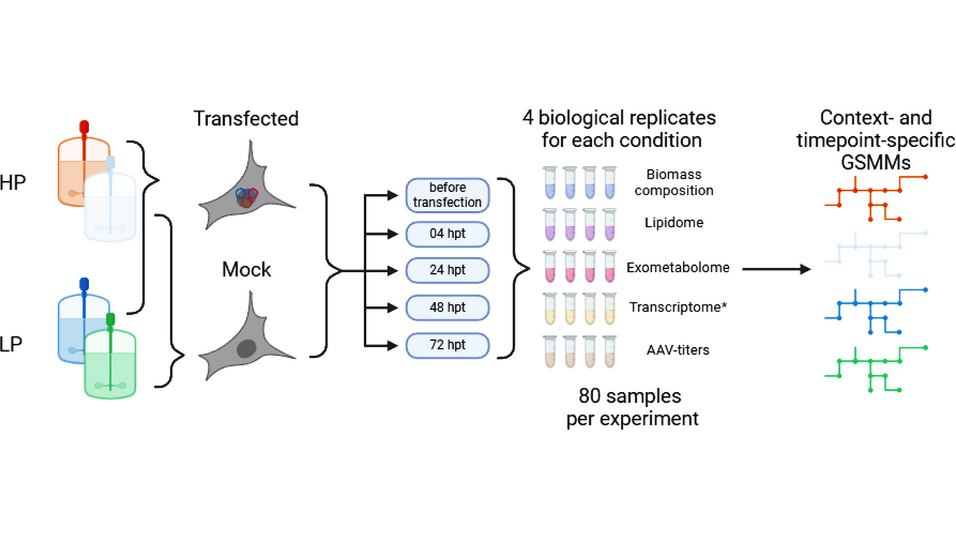
This study presents a comprehensive multi-omics analysis of two HEK293 strains under good manufacturing practice conditions, focusing on the metabolic and cellular responses during AAV production. The investigation included lipidomic, exometabolomic, and transcriptomic profiling across different conditions and time points. Genome-scale metabolic models (GSMMs) were reconstructed for these strains to elucidate metabolic shifts and identify potential bottlenecks in AAV production.
Notably, the study revealed significant differences between a High-producing (HP) and a Low-producing (LP) HEK293 strains, highlighting pseudohypoxia in the LP strain. Key findings include the identification of hypoxia-inducible factor 1-alpha (HIF-1 ) as a critical regulator in the LP strain, linking pseudohypoxia to poor AAV productivity.
Inhibition of HIF-1 resulted in immediate cessation of cell growth and a 2.5-fold increase in viral capsid production, albeit with a decreased number of viral genomes, impacting the full-to-empty particle ratio. This trade-off is significant because it highlights a key challenge in AAV production: achieving a balance between capsid assembly and genome packaging to optimize the yield of functional viral vectors. Overall this suggests that while HIF-1 inhibition enhances capsid assembly, it simultaneously hampers nucleotide synthesis via the pentose phosphate pathway (PPP), necessary for nucleotide synthesis, and therefore for AAV genome replication.