Published in Microb Cell Fact 22, 242. 2023.
doi.org/10.1186/s12934-023-02248-2
See the code on GitHub.
Abstract
Plasmid DNA (pDNA) is a key biotechnological product whose importance became apparent in the last years due to its role as a raw material in the messenger ribonucleic acid (mRNA) vaccine manufacturing process. In pharmaceutical production processes, cells need to grow in the defined medium in order to guarantee the highest standards of quality and repeatability. However, often these requirements result in low product titer, productivity, and yield.
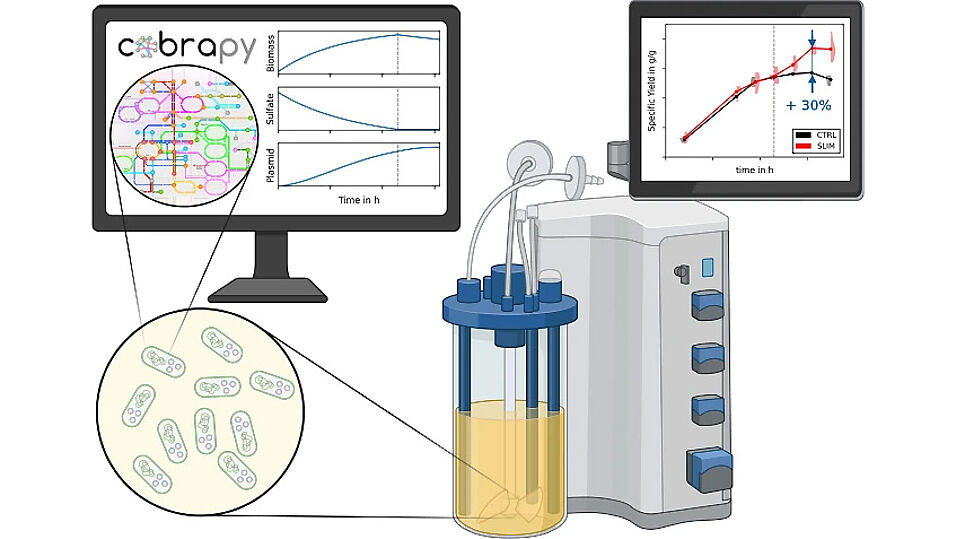
In this study, we used constraint-based metabolic modeling to optimize the average volumetric productivity of pDNA production in a fed-batch process. We identified a set of 13 nutrients in the growth medium that are essential for cell growth but not for pDNA replication. When these nutrients are depleted in the medium, cell growth is stalled and pDNA production is increased, raising the specific and volumetric yield and productivity. To exploit this effect we designed a three-stage process (1. batch, 2. fed-batch with cell growth, 3. fed-batch without cell growth). The transition between stage 2 and 3 is induced by sulfate starvation. Its onset can be easily controlled via the initial concentration of sulfate in the medium.
We validated the decoupling behavior of sulfate and assessed pDNA quality attributes (supercoiled pDNA content) in E. coli with lab-scale bioreactor cultivations. The results showed an increase in supercoiled pDNA to biomass yield by 29% upon limitation of sulfate.
In conclusion, even for routinely manufactured biotechnological products such as pDNA, simple changes in the growth medium can significantly improve the yield and quality.
Highlights
- Genome-scale metabolic models predict growth decoupling strategies
- Sulfate limitation decouples cell growth from pDNA production
- Sulfate limitation increases the specific supercoiled pDNA yield by 33% and the volumetric productivity by 13%.
- We propose that sulfate limitation improves the biosynthesis of over 25% of naturally secreted products in E. coli.